Learn The Best Ways to Work With Dissimilar Metals
AEP Span’s steel-based products, painted and bare, feature a metallic-coating that provides long-lasting resistance from corrosion. This coating is applied during the steel production process prior to the application of paint. Metallic coatings consist of either zinc (sold as TruZinc® or galvanized) or a specialty mix of aluminum and zinc (sold as ZINCALUME®). Care must be taken during the design and installation phase of metallic-coated products to avoid the unintentional creation of galvanic corrosion. This rapid form of corrosion is induced when metals of varying types (dissimilar metals) are installed in direct contact with one another in a corrosive environment.
GALVANIC CORROSION
Galvanic or bi-metallic corrosion is a reaction in which one metal will sacrifice itself (or dissolve) to protect the different, less active metal, leading to visible corrosion. Galvanic corrosion occurs between dissimilar metals in certain corrosive environments and typically requires three criteria in which to occur:
- Two or more dissimilar metals,
- Metal to metal contact, and,
- Both metals to reside in the same conducting solution/ corrosive environment, i.e. salt air or water.
Metals can be viewed as active or noble based on their position on the galvanic scale, simplified in Table 1 below. The further apart these metals are on this scale, the greater the potential for a reaction between the metals. In addition to the dissimilarity of the metals, the severity of the environment will influence the potential for galvanic corrosion. Common factors influencing environmental severity can include direct proximity to salt water or chemical spray, the frequency of breaking surf (which generates airborne salt-laden particulates), and how frequently the surface is rinsed by rainfall.
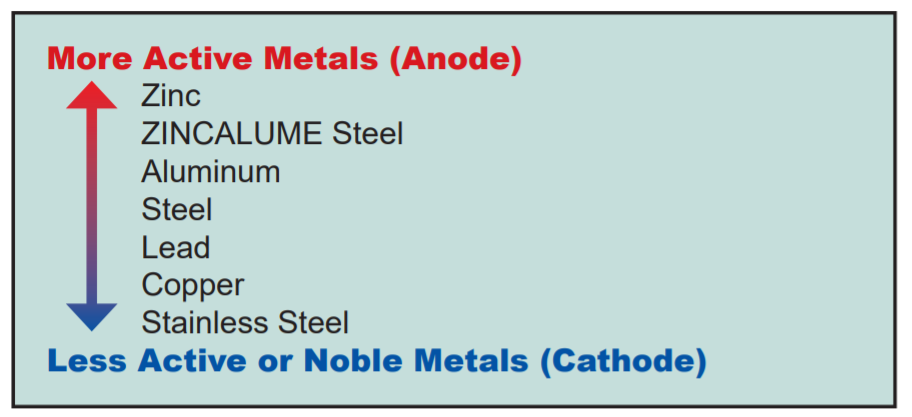
Galvanic Series of Metallic Activity
IN APPLICATION
Installations featuring galvanic corrosion are commonly attributed to improper fastener and accessory selection. This includes the use of stainless steel and copper products in direct contact with metallic-coated metal roofing and siding profiles.
Stainless-Steel Hardware (Clips, Fasteners, etc)
Galvanic corrosion can be caused by stainless-steel fasteners, rivets, light fixtures, window frames and other accessories recessed in metallic-coated panels in corrosive environments. Initially this may appear as the apparent bubbling of the painted metal roof or siding surface around the stainless-steel fixture. Often falsely identified as a paint failure, this bubbling is the steel underneath corroding, resulting in the loss of paint adhesion. AEP Span’s stainless-steel hardware offering, such as clips and fasteners, are not intended to be used with ZINCALUME coated steel profiles in corrosive environments. In these environments stainless steel components should be used in conjunction with profiles fabricated out of aluminum.
Copper and Copper Treated Lumber
Metallic coated steel will experience accelerated corrosion when it is in contact with copper, this includes copper treated lumber. Leeching of copper from treated materials, such as through water run-off from rainfall or condensation, can result in corrosion without direct metal to metal contact. In these instances, the protective oxide film which naturally forms on aluminum-zinc coatings is broken down by copper in localized areas. Pitting corrosion ensues which is a highly accelerated form of attack. Zinc coatings are not generally subject to pitting but will demonstrate the effects of galvanic corrosion when installed in direct contact with copper.
AEP Span recommends avoiding the use of copper where galvanic corrosion may be a concern. If the use of copper cannot be avoided, AEP Span recommends that insulated copper or a protective coating over the copper be used to prevent exposure. Any electrical terminals that utilize exposed copper should also be sealed with shrink tape or similar method This includes but is not limited to grounding wire for Photo Voltaic Arrays, Lightning Protection, and Pipe Penetrations.
Galvanized Steel and ZINCALUME Coated Steel
Unpainted galvanized steel must not be used for roofing or rainwater goods (including valleys and gutters) to collect water runoff from ZINCALUME Steel or other more noble metals. ZINCALUME Steel and painted ZINCALUME Steel can be used to collect water from galvanized catchment material. Irrespective of galvanic corrosion considerations, ZINCALUME Steel panels & gutters will typically give a longer service life than traditional galvanized steel. Neither product should be used as catchment material for roofs featuring more noble metals such as aluminum or copper.
WARRANTY IMPLICATIONS AND REMEDY
Product deterioration or corrosion created by the improper installation of dissimilar metals will void any warranty supplied by AEP Span. This includes product performance, paint system, and corrosion warranties.
If galvanic corrosion is identified, this issue must be rectified as promptly as possible due to the rapid rate at which it can spread. Remedies may include the replacement of the panels impacted by corrosion and the substitution or relocation of the associated dissimilar metals. For more information on the identification or rectification of galvanic corrosion, please contact AEP Span.
Download the Technical Bulletin HERE in PDF form.
Did you find this article helpful?


















































































